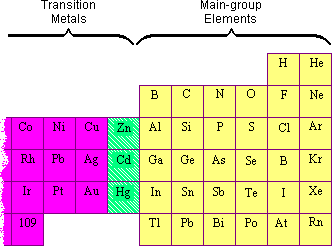
Excellent! Why Is Zinc Not A Transition Metal
The definition of a transition metal is that it must have an incomplete d sub-level in one or more of is oxidation states. A transition metal is one that forms one or more stable ions which have incompletely filled d orbitals.
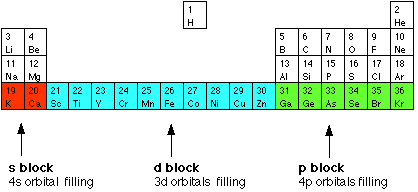
8 1 Chemistry Of The Transition Metals Chemistry Libretexts
Zinc has completely filled d-orbital and should thus not be a metal for transition.
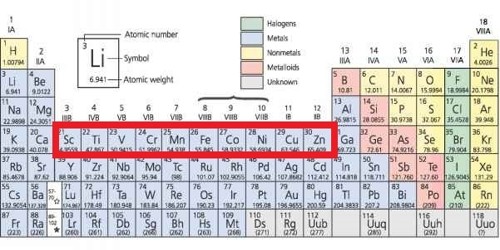
Why is zinc not a transition metal. The electron configuration of zinc is Ar4s 2 3d 10. Why zinc is not a transition metal. The only oxidative state which zinc has is Zn 2 in which its configuration is Ar 4s 0 3d 10 as the 4s sub-level empties first.
Why is zinc not considered a transition metal. On the basis of this definition. The name was assigned to the transition metals because they had a position in the main group elements.

The General Features Of Transition Metal Chemistry Ppt Download

Explain Why Zinc Is Not Rgardede As A Transition Element

Part 4 D Block Elements First Row Adapted From Mrs D Dogancay Ppt Download

Chapter 15 Transition Metals 15 1 General Properties Of Transition Metals 15 2 Complex Formation And The Shape Of Complex Ions 15 3 Coloured Ions Ppt Download

All Transition Elements Are D Block Elements But D Block Elements Are Not Transition Qs Study

Why Zn Is Not A Transition Metal
Lesson Explainer Transition Metals Nagwa

Topic 13 Transition Elements Chemistrycorner